Call us: +44 870 8 200 310
Or write: experts@regartis.com
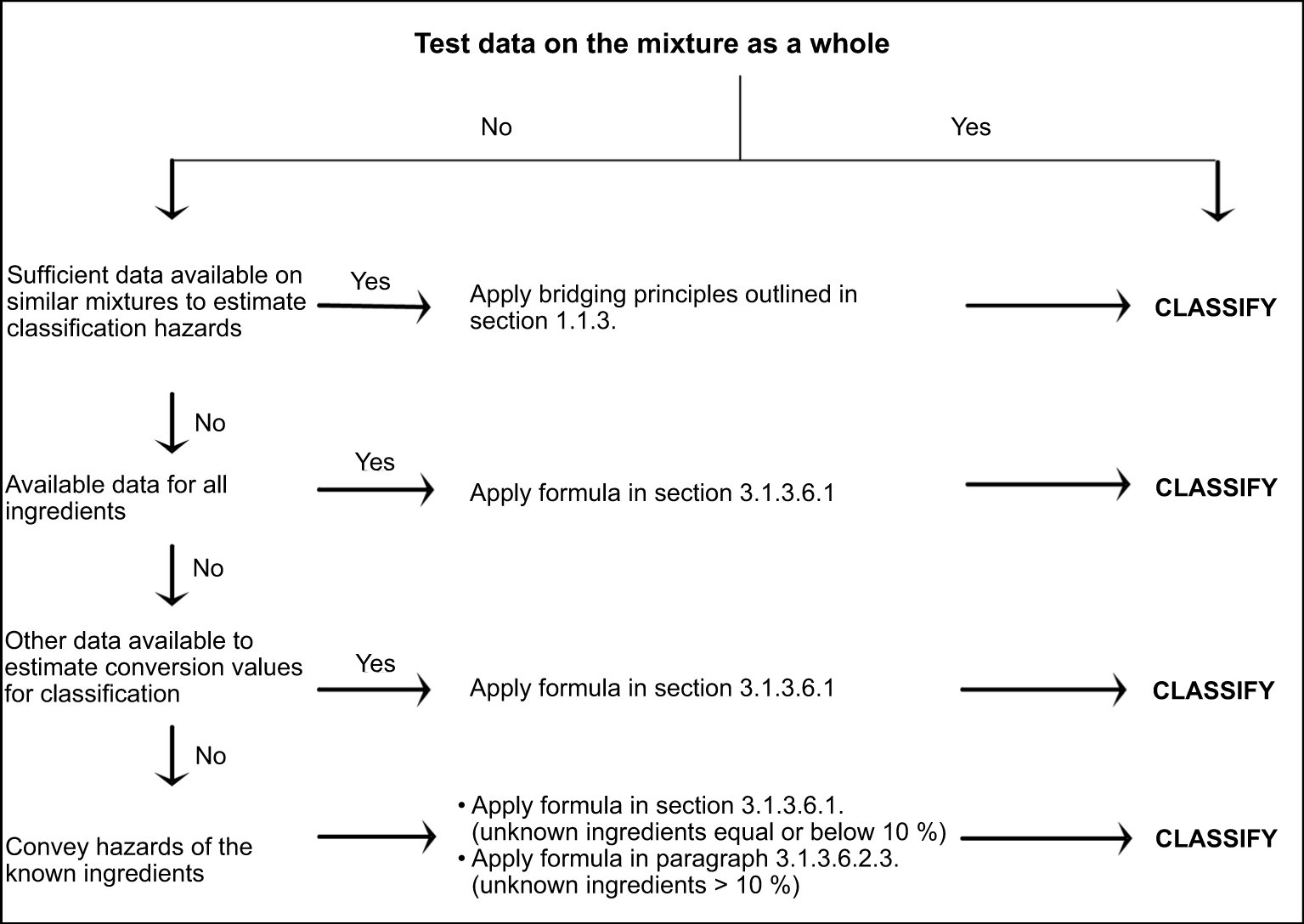
3.1.3.: Criteria for classification of mixtures as acutely toxic
Figure 3.1.1
Tiered approach to classification of mixtures for acute toxicity
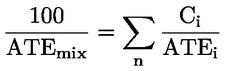
|
Ci |
= |
concentration of ingredient i ( % w/w or % v/v) |
|
i |
= |
the individual ingredient from 1 to n |
|
n |
= |
the number of ingredients |
|
ATEi |
= |
Acute Toxicity Estimate of ingredient i. |
This includes evaluation of:
This approach generally requires substantial supplemental technical information, and a highly trained and experienced expert (expert judgement, see section 1.1.1), to reliably estimate acute toxicity. If such information is not available, proceed to paragraph 3.1.3.6.2.3.
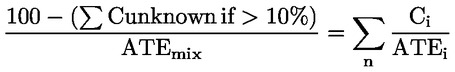
Table 3.1.2
Conversion from experimentally obtained acute toxicity range values (or acute toxicity hazard categories) to acute toxicity point estimates for use in the formulas for the classification of mixtures
|
Exposure routes |
Classification Category or experimentally obtained acute toxicity range estimate |
Converted acute toxicity point estimate (see Note 1) |
|
Oral (mg/kg bodyweight) |
0 < Category 1 ≤ 5 5 < Category 2 ≤ 50 50 < Category 3 ≤ 300 300 < Category 4 ≤ 2 000 |
0,5 5 100 500 |
|
Dermal (mg/kg bodyweight) |
0 < Category 1 ≤ 50 50 < Category 2 ≤ 200 200 < Category 3 ≤ 1 000 1 000 < Category 4 ≤ 2 000 |
5 50 300 1 100 |
|
Gases (ppmV) |
0 < Category 1 ≤ 100 100 < Category 2 ≤ 500 500 < Category 3 ≤ 2 500 2 500 < Category 4 ≤ 20 000 |
10 100 700 4 500 |
|
Vapours (mg/l) |
0 < Category 1 ≤ 0,5 0,5 < Category 2 ≤ 2,0 2,0 < Category 3 ≤ 10,0 10,0 < Category 4 ≤ 20,0 |
0,05 0,5 3 11 |
|
Dust/mist (mg/l) |
0< Category 1 ≤ 0,05 0,05 < Category 2 ≤ 0,5 0,5 < Category 3 ≤ 1,0 1,0 < Category 4 ≤ 5,0 |
0,005 0,05 0,5 1,5 |
Note 1

