Call us: +44 870 8 200 310
Or write: experts@regartis.com
Languages
Regulations
- Preamble
- TITLE I / GENERAL ISSUES
- TITLE II / HAZARD CLASSIFICATION
-
TITLE III / HAZARD...
-
CHAPTER 1 / Content of the label
- Article 17 / General rules
- Article 18 / Product identifiers
- Article 19 / Hazard pictograms
- Article 20 / Signal words
- Article 21 / Hazard statements
- Article 22 / Precautionary statements
- Article 23 / Derogations...
- Article 24 / Request for use...
- Article 25 / Supplemental...
- Article 26 / Principles of...
- Article 27 / Principles of...
- Article 28 / Principles of...
- Article 29 / Exemptions from...
- Article 30 / Updating...
- CHAPTER 2 / Application of labels
-
CHAPTER 1 / Content of the label
- TITLE IV / PACKAGING
- TITLE V / HARMONISATION OF...
- TITLE VI / COMPETENT...
-
TITLE VII / COMMON AND FINAL...
- Article 48 / Advertisement
- Article 49 / Obligation to...
- Article 50 / Tasks of the Agency
- Article 51 / Free movement clause
- Article 52 / Safeguard clause
- Article 53 / Adaptations to...
- Article 54 / Committee procedure
- Article 55 / Amendments to...
- Article 56 / Amendments to...
- Article 57 / Amendments to...
- Article 58 / Amendments to...
- Article 59 / Amendments to...
- Article 60 / Repeal
- Article 61 / Transitional provisions
- Article 62 / Entry into force
-
ANNEX I / CLASSIFICATION AND...
- 1. / PART 1: GENERAL...
-
2. / PART 2: PHYSICAL HAZARDS
- 2.1. / Explosives
- 2.2. / Flammable gases...
- 2.3. / Aerosols
- 2.4. / Oxidising gases
- 2.5. / Gases under pressure
- 2.6. / Flammable liquids
- 2.7. / Flammable solids
- 2.8. / Self-reactive...
- 2.9. / Pyrophoric liquids
- 2.10. / Pyrophoric solids
- 2.11. / Self-heating...
- 2.12. / Substances and...
- 2.13. / Oxidising liquids
- 2.14. / Oxidising solids
- 2.15. / Organic peroxides
- 2.16. / Corrosive to metals
- 3. / PART 3: HEALTH HAZARDS
- 4. / PART 4: ENVIRONMENTAL HAZARDS
- 5. / PART 5: ADDITIONAL HAZARDS
-
ANNEX II / SPECIAL RULES FOR...
- 1. / PART 1: SUPPLEMENTAL...
-
2. / PART 2: SPECIAL RULES...
- 2.1. / Mixtures containing lead
- 2.2. / Mixtures containing...
- 2.3. / Cements and cement mixtures
- 2.4. / Mixtures containing...
- 2.5. / Mixtures containing...
- 2.6. / Mixtures sold to the...
- 2.7. / Mixtures containing...
- 2.8. / Mixtures containing...
- 2.9. / Liquid mixtures...
- 2.10. / Mixtures not...
- 2.11 / Aerosols
- 3. / PART 3: SPECIAL RULES...
- 4. / PART 4: SPECIAL RULE...
- 5. / PART 5: LIST OF...
- ANNEX III / LIST OF HAZARD...
- ANNEX IV / LIST OF...
- ANNEX V / HAZARD PICTOGRAMS
- ANNEX VI / Harmonised...
- ANNEX VII / Translation...
2.3.4.: Additional Classification Considerations
For a composite aerosol formulation, the chemical heat of combustion is the summation of the weighted heats of combustion for the individual components, as follows:
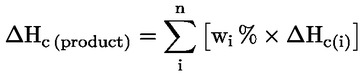
where:
|
ΔΗc |
= |
chemical heat of combustion (kJ/g); |
|
wi % |
= |
mass fraction of component i in the product; |
|
ΔΗc(i) |
= |
specific heat of combustion (kJ/g)of component i in the product. |
The chemical heats of combustion can be found in the literature, calculated or determined by tests (see ASTM D 240 as amended — Standard Test Methods for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter, EN/ISO 13943 as amended, 86.l to 86.3 — Fire safety — Vocabulary, and NFPA 30B as amended — Code for the Manufacture and Storage of Aerosol Products).

